A clinical trial conducted in Eastern Africa has discovered a shorter, safer, and more effective treatment for post-kala-azar dermal leishmaniasis (PKDL), a stigmatizing skin disease.
According to researchers, the new treatment which reduces both hospitalization days by over 50pc and the safety risks associated with long antimonial therapy (injectables) is child-friendly.
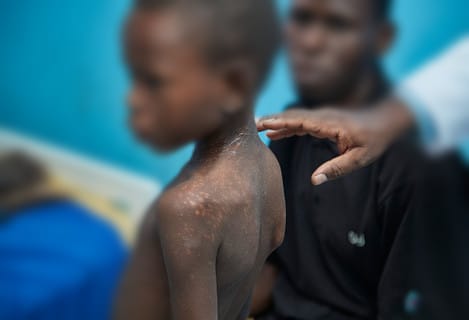
PKDL can develop after someone has been treated for visceral leishmaniasis (VL), also known as kala-azar or black fever which is a life-threatening disease.
Most common in Eastern Africa and South Asia, the condition starts with a rash around the mouth and can spread to the arms and upper body, and eventually to the entire body, depending on severity.
In Sudan, nearly 20pc of VL patients will develop PKDL within six months post-treatment, the highest rate worldwide.
The trial was conducted in Sudan by the non-profit medical research organization Drugs for Neglected Diseases Initiative (DNDi) and the Institute of Endemic Diseases at the University of Khartoum, whose results were published in PLOS Neglected Tropical Diseases.
Phase II trial
The Phase II trial, testing two treatment arms, began in 2018 in Doka, Sudan, with nearly 90% of participants being 12 years or younger. Arm 1 received a combination of oral miltefosine and injectable paromomycin (MF+PM) for 42 days.
However, they only needed to stay in the hospital for 14 days during the PM treatment while starting the oral miltefosine simultaneously with the injectable.
After leaving the hospital, they continued with the oral treatment at home. At the 12-month follow-up visit, 98% of the participants had achieved a complete cure, including those with moderate and mild PKDL.
Arm 2 received a regimen of miltefosine and injectable liposomal amphotericin B (MF+LAmB) for 28 days, staying in the hospital for only 7 days until the LAmB treatment ended and continuing the oral treatment at home after hospital discharge.
“This group had an 80% cure rate, with MF+LAmB proving to be a good treatment alternative,” DNDi said in a statement.
Currently, the standard treatment for PKDL is sodium stibogluconate (SSG), an injectable drug given for a lengthy 60-90 days that carries life-threatening toxicity when used for an extended period and requires hospital admission as it must be administered under close supervision.
“Treatment for PKDL in Sudan is currently only recommended for patients with severe or persistent disease, mainly because SSG is prolonged, toxic, and expensive,”, said Professor Ahmed Musa, Senior Investigator for Leishmaniasis from the Institute of Endemic Diseases, University of Khartoum.
“But we have now found a safer and better treatment option where patients only need to be admitted to hospital for 14 days and then complete the oral treatment at home. This makes it more patient-friendly, which is important since most people affected by this terrible disease are children” he added.
World Health Organization insists detecting and treating PKDL is crucial for eliminating VL as a public health problem.
People treated for PKDL can help prevent new cases since the sandflies that transmit VL get infected when they feed on PKDL lesions.
One of the targets in the recently launched VL elimination framework for Eastern Africa is to ensure that all PKDL cases are detected, reported, and managed by 2030. The results of the clinical trial led in Sudan therefore support VL elimination efforts in the region.
‘For a long time, patients with PKDL in Eastern Africa have been left behind by medical research because the disease is not considered life-threatening. Many have had to endure not only stigma but expensive, lengthy treatments exposing them to toxicity,’ said Dr Fabiana Alves, Director of the Leishmaniasis Cluster at DNDi. ‘But this new, shorter, and better treatment will improve the lives of these neglected patients and also help reduce VL transmission on our road to elimination.’
The results of this study will provide evidence for policymakers to guide recommendations for new and more patient-friendly treatments, allowing people with PKDL to benefit from these findings.
Financial support for this study was provided by the World Health Organization – Special Programme for Research and Training in Tropical Diseases (WHO-TDR); the French Development Agency (AFD); Médecins Sans Frontières; the UK International Development; and the Swiss Agency for Development and Cooperation (SDC).